Sanger Sequencing Confirmation
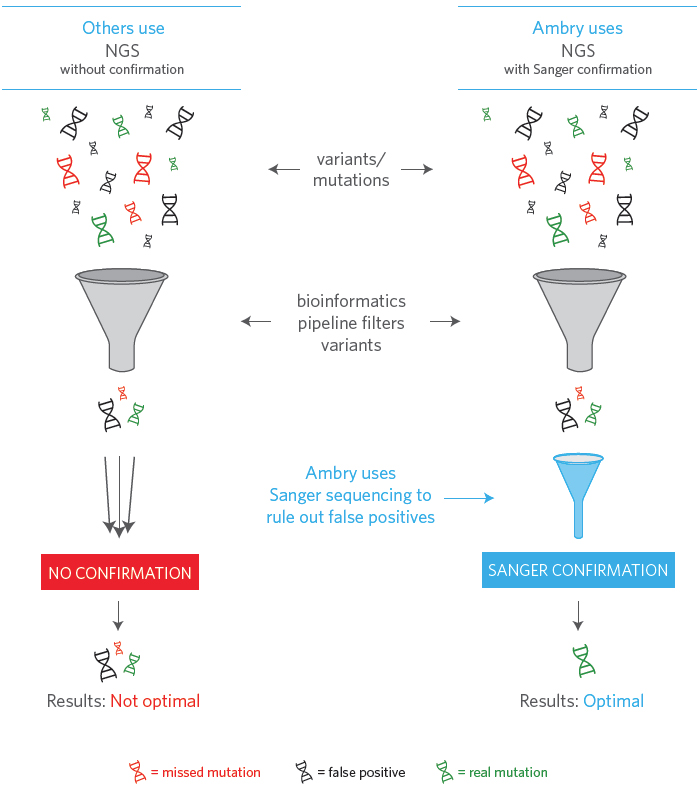
Many labs validate their tests based on certain limited studies. Ambry places value above all else through quality lab processes, confirmatory testing, and advanced technology. This is to ensure that the results healthcare providers receive are as accurate and informative as possible. That’s why we led the largest study of its kind (20,000 cases) guiding us to use additional confirmatory testing when we saw specific well-defined thresholds, reducing the chance of delivering a false positive patient report or one that misses real mutations. (Mu et al., November 2016)
NGS without Sanger confirmation means sensitivity and specificity are not optimized. 1.3% of mutations identified would be false positives; controlling a bioinformatics pipeline to eliminate these would result in missing 2% of real mutations.1 The more accurate reports we provide, the more insight healthcare providers will have to better treat their patients.
Ambry’s high quality shows in every step
Ambry is a pioneer in NGS, having adopted the technology in 2007 and integrated it into operations since 2010. We have processed more than 1.5 million tests by NGS and learned about the limitations in the technology.
By maintaining the most sensitive bioinformatics pipeline available along with Sanger sequencing NGS variants, Ambry ensures the removal of false positives while keeping accuracy as high as possible. This makes sure that only the real mutations are reported to clinicians, who are then making medical decisions with and for their patients.
References:
1. Mu W, Lu H-M, Chen J, Li S, Elliott AM. Sanger confirmation is required to achieve optimal sensitivity and specificity in next-generation sequencing panel testing. The Journal of Molecular Diagnostics. November 2016 18(6):923-32.